Clinical Biochemistry
Confirmation of Mband (Identifying Artefacts in SPE).
Serum Protein Electrophoresis (SPE) is a laboratory technique used to separate serum protein into 5 distinct fractions ( albumin, alpha 1, alpha 2, beta, gamma ). It can be used to measure the concentration of the serum protein as well. It can also be used to determine absence of normal proteins and to identify presence of abnormal proteins. SPE is normally requested for myeloma monitoring. It is often requested to identify M-band.
M-band, is a protein which is either immunoglobin or immunoglobin light chains. It will form a distinct band often at the gamma region. M-band is due to excessive production of immunoglobins due to diseases such as multiple myeloma, Monoclonal Gammopathy of Undetermined Significance, chronic lymphocytic leukemia, etc.
However, it is critical to ensure that the M-band seen on the SPE film is of an immunoglobin class. Sometimes, it may be due to artefacts. One good example of an artefact is fibrin frmo a plasma sample which looks like a M-band.
We therefore run immunofixation (IFE) to identify the immunoglobin class of the M-band.
Here are some pictures to illustrate what i have just said.
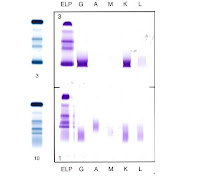 As you can see, the top blue bands are SPE of a sample which has a M-band. Confirmation from the IFE (purple) shows that the immunoglobin class is IgG/Kappa. However, the bottom blue bands of the SPE, is a plasma sample containing fibrin. It also has a distinct band in the gamma region which looks like a M-band. However when IFE is done, you notice that no distinct band of immunoglobin class appears. Therefore we understand that it is an artefact.
As you can see, the top blue bands are SPE of a sample which has a M-band. Confirmation from the IFE (purple) shows that the immunoglobin class is IgG/Kappa. However, the bottom blue bands of the SPE, is a plasma sample containing fibrin. It also has a distinct band in the gamma region which looks like a M-band. However when IFE is done, you notice that no distinct band of immunoglobin class appears. Therefore we understand that it is an artefact.
